- Formula: F
- Molecular weight: 18.9984032
- IUPAC Standard InChI:
- InChI=1S/F
- Download the identifier in a file.
- IUPAC Standard InChIKey:YCKRFDGAMUMZLT-UHFFFAOYSA-N
- CAS Registry Number: 14762-94-8
- Chemical structure:
This structure is also available as a 2d Mol file - Permanent link for this species. Use this link for bookmarking this speciesfor future reference.
- Information on this page:
- Other data available:
- Data at other public NIST sites:
- Options:
Data at NIST subscription sites:
Fluorine has higher ionization energy than iodine because the size of fluorine is smaller than the iodine. This means that the shielding effect is less for fluorine. Therefore, the nucleus attracts more valence electrons in fluorine than in iodine. Stay tuned with BYJU’S to learn more about other concepts such as ionization energy. Ionization energy determinations. IE (eV) Method., High Resolution Determination of the Electron Affinity of Fluorine and Bromine using Crossed Ion. It is because of the shielding effect that the ionization energy decreases from top to bottom within a group. From this trend, Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy (with the exception of Helium and Neon).
NIST subscription sites provide data under theNIST Standard ReferenceData Program, but require an annual fee to access.The purpose of the fee is to recover costs associatedwith the development of data collections included insuch sites. Your institution may already be a subscriber.Follow the links above to find out more about the datain these sites and their terms of usage.
F atom is smaller than Cl atom Therefore the effective nuclear attraction force on the outermost electrons in F is higher than that in Cl. So lesser energy must be provided to remove the outermost electron in Cl when compared to that with Fl. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. X + energy → X + + e −. Where X is any atom or molecule capable of being ionized, X + is that atom or molecule with an electron removed (positive ion), and e − is the removed electron. A Fluorine atom, for example.
Gas phase ion energetics data
Go To:Top, References, Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
Data evaluated as indicated in comments:
HL - Edward P. Hunter and Sharon G. Lias
L - Sharon G. Lias
Data compiled as indicated in comments:
B - John E. Bartmess
LL - Sharon G. Lias and Joel F. Liebman
LBLHLM - Sharon G. Lias, John E. Bartmess, Joel F. Liebman, John L. Holmes, Rhoda D. Levin, and W. Gary Mallard
LLK - Sharon G. Lias, Rhoda D. Levin, and Sherif A. Kafafi
RDSH - Henry M. Rosenstock, Keith Draxl, Bruce W. Steiner, and John T. Herron
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| IE (evaluated) | 17.42282 | eV | N/A | N/A | L |
| Quantity | Value | Units | Method | Reference | Comment |
| Proton affinity (review) | 340.1 | kJ/mol | N/A | Hunter and Lias, 1998 | HL |
| Quantity | Value | Units | Method | Reference | Comment |
| Gas basicity | 315.1 | kJ/mol | N/A | Hunter and Lias, 1998 | HL |
Electron affinity determinations
| EA (eV) | Method | Reference | Comment |
|---|---|---|---|
| 3.401191 ± 0.000026 | LPD | Blondel, Delsart, et al., 2001 | Given: 3.4011895(25) eV, or 27432.446(19) cm-1, or 78.433266(577) kcal/mol; B |
| 3.40625 | D-EA | Drechsler, Boesl, et al., 1997 | Given: 129557.1±0.9 cm-1 at 0K (370.422±0.003). Corr to 298 with data in Gurvich, Veyts, et al. (=371.321); B |
| 3.40118 | LPD | Blondel, Cacciani, et al., 1989 | Reported: 3.401190±0.000004 eV. acidity includes 0.9 kcal 0 to 298 K correction.; B |
| 3.4480 ± 0.0050 | N/A | Berry and Reimann, 1963 | B |
| 3.48526 | N/A | Check, Faust, et al., 2001 | FeCl3-; ; ΔS(EA)=5.0; B |
Ionization energy determinations
Ionization Energy Of Fluorine 2
| IE (eV) | Method | Reference | Comment |
|---|---|---|---|
| 17.2 | EI | Veljkovic, Neskovic, et al., 1992 | LL |
| 17.42282 | EVAL | Lide, 1992 | LL |
| 17.423 | S | Kelly, 1987 | LBLHLM |
| 17.418 | S | Palenius, Huffman, et al., 1978 | LLK |
| 17.431 | S | Palenius, Huffman, et al., 1978 | LLK |
| 17.47 ± 0.02 | PE | De Leeuw, Mooyman, et al., 1978 | LLK |
| 17.42282 | S | Moore, 1970 | RDSH |

Anion protonation reactions
+ =
By formula: F- + H+ = HF
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | 1555. ± 5. | kJ/mol | AVG | N/A | Average of 6 out of 7 values; Individual data points |
| Quantity | Value | Units | Method | Reference | Comment |
| ΔrG° | 1530.0 ± 0.75 | kJ/mol | H-TS | Blondel, Delsart, et al., 2001 | gas phase; Given: 3.4011895(25) eV, or 27432.446(19) cm-1, or 78.433266(577) kcal/mol; B |
| ΔrG° | 1529.4 | kJ/mol | H-TS | Martin and Hepburn, 2000 | gas phase; Given: 371.334±0.003 kcal/mol (corr to 298K with data from Wagman, Evans, et al., 1982).H(0K)=370.422±0.003; B |
| ΔrG° | 1530.0 ± 0.75 | kJ/mol | H-TS | Blondel, Cacciani, et al., 1989 | gas phase; Reported: 3.401190±0.000004 eV. acidity includes 0.9 kcal 0 to 298 K correction.; B |
| ΔrG° | 1529. ± 8.4 | kJ/mol | IMRE | Bierbaum, Schmidt, et al., 1981 | gas phase; B |
| ΔrG° | 1503.7 | kJ/mol | N/A | Check, Faust, et al., 2001 | gas phase; FeCl3-; ; ΔS(EA)=5.0; B |
References
Go To:Top, Gas phase ion energetics data, Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
Hunter and Lias, 1998
Hunter, E.P.; Lias, S.G.,Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update,J. Phys. Chem. Ref. Data, 1998, 27, 3, 413-656, https://doi.org/10.1063/1.556018. [all data]
Blondel, Delsart, et al., 2001
Blondel, C.; Delsart, C.; Goldfarb, F.,Electron spectrometry at the mu eV level and the electron affinities of Si and F,J. Phys. B: Atom. Mol. Opt. Phys., 2001, 34, 9, L281-L288, https://doi.org/10.1088/0953-4075/34/9/101. [all data]
Drechsler, Boesl, et al., 1997
Drechsler, G.; Boesl, U.; Bassmann, C.; Schlag, E.W.,Mass selected anion-zero kinetic energy photoelectron spectroscopy (anion-ZEKE): Ground and low excited states of FeO,J. Chem. Phys., 1997, 107, 7, 2284-2291, https://doi.org/10.1063/1.474622. [all data]
Gurvich, Veyts, et al.
Gurvich, L.V.; Veyts, I.V.; Alcock, C.B.,Hemisphere Publishing, NY, 1989, V. 1 2, Thermodynamic Properties of Individual Substances, 4th Ed. [all data]
Blondel, Cacciani, et al., 1989
Blondel, C.; Cacciani, P.; Delsart, C.; Trainham, R.,High Resolution Determination of the Electron Affinity of Fluorine and Bromine using Crossed Ion and Laser Beams,Phys. Rev. A, 1989, 40, 7, 3698, https://doi.org/10.1103/PhysRevA.40.3698. [all data]
Berry and Reimann, 1963
Berry, R.S.; Reimann, C.W.,Absorption Spectrum of Gaseous Fluoride and the Electron Affinities of the Halogen Atoms,J. Chem. Phys., 1963, 38, 7, 1540, https://doi.org/10.1063/1.1776916. [all data]
Check, Faust, et al., 2001
Check, C.E.; Faust, T.O.; Bailey, J.M.; Wright, B.J.; Gilbert, T.M.; Sunderlin, L.S.,Addition of Polarization and Diffuse Functions to the LANL2DZ Basis Set for P-Block Elements,J. Phys. Chem. A,, 2001, 105, 34, 8111, https://doi.org/10.1021/jp011945l. [all data]
What Is The Ionization Energy Of Fluorine
Veljkovic, Neskovic, et al., 1992
Veljkovic, M.V.; Neskovic, O.M.; Zmbov, K.F.,Mass spectrometric study of the thermal decomposition of F2,J. Serb. Chem. Soc., 1992, 57, 753. [all data]
Lide, 1992
Lide, D.R. (Editor),Ionization potentials of atoms and atomic ionsin Handbook of Chem. and Phys., 1992, 10-211. [all data]
Kelly, 1987
Kelly, R.L.,Atomic and ionic spectrum lines of hydrogen through kryton,J. Phys. Chem. Ref. Data, 1987, 16. [all data]
Palenius, Huffman, et al., 1978
Palenius, H.P.; Huffman, R.E.; Larrabee, J.C.; Tanaka, Y.,The absorption spectrum of fluorine F I observed with the helium continuum,J. Opt. Soc. Am., 1978, 68, 1564. [all data]
De Leeuw, Mooyman, et al., 1978
De Leeuw, D.M.; Mooyman, R.; De Lange, C.A.,He(I) photoelectron spectroscopy of halogen atoms,Chem. Phys. Lett., 1978, 54, 231. [all data]
Moore, 1970
Moore, C.E.,Ionization potentials and ionization limits derived from the analyses of optical spectra,Natl. Stand. Ref. Data Ser., (U.S. Natl. Bur. Stand.), 1970, 34, 1. [all data]
Martin and Hepburn, 2000
Martin, J.D.D.; Hepburn, J.W.,Faraday Disc. Chem. Soc., 2000, 115, 416. [all data]
Wagman, Evans, et al., 1982
Wagman, D.D.; Evans, W.H.; Parker, V.B.; Schumm, R.H.; Halow, I.; Bailey, S.M.; Churney, K.L.; Nuttall, R.L.,The NBS Tables of Chemical Thermodynamic Properties (NBS Tech Note 270),J. Phys. Chem. Ref. Data, Supl. 1, 1982, 11. [all data]
Bierbaum, Schmidt, et al., 1981
Bierbaum, V.M.; Schmidt, R.J.; DePuy, C.H.; Mead, R.H.; Schulz, P.A.; Lineberger, W.C.,Reactions of carbanions with triplet and singlet molecular oxygen,J. Am. Chem. Soc., 1981, 103, 6262. [all data]
Notes
Go To:Top, Gas phase ion energetics data, References
- Symbols used in this document:
EA Electron affinity IE (evaluated) Recommended ionization energy ΔrG° Free energy of reaction at standard conditions ΔrH° Enthalpy of reaction at standard conditions - Data from NIST Standard Reference Database 69:NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST)uses its best efforts to deliver a high quality copy of theDatabase and to verify that the data contained therein havebeen selected on the basis of sound scientific judgment.However, NIST makes no warranties to that effect, and NISTshall not be liable for any damage that may result fromerrors or omissions in the Database.
- Customer supportfor NIST Standard Reference Data products.
The halogens are located on the left of the noble gases on the periodic table. These five toxic, non-metallic elements make up Group 17 of the periodic table and consist of: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). Although astatine is radioactive and only has short-lived isotopes, it behaves similar to iodine and is often included in the halogen group. Because the halogen elements have seven valence electrons, they only require one additional electron to form a full octet. This characteristic makes them more reactive than other non-metal groups.
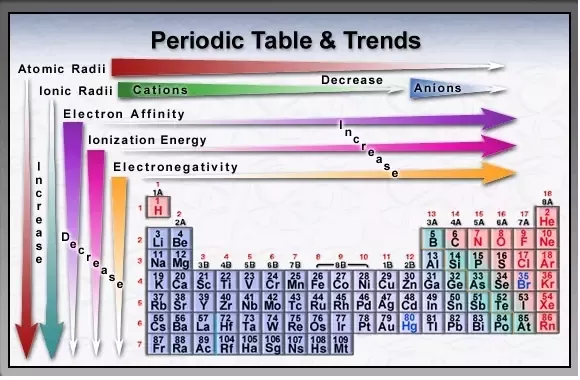
Introduction
Halogens form diatomic molecules (of the form X2, where X denotes a halogen atom) in their elemental states. The bonds in these diatomic molecules are non-polar covalent single bonds. However, halogens readily combine with most elements and are never seen uncombined in nature. As a general rule, fluorine is the most reactive halogen and astatine is the least reactive. All halogens form Group 1 salts with similar properties. In these compounds, halogens are present as halide anions with charge of -1 (e.g. Cl-, Br-, etc.). Replacing the -ine ending with an -ide ending indicates the presence of halide anions; for example, Cl- is named 'chloride.' In addition, halogens act as oxidizing agents—they exhibit the property to oxidize metals. Therefore, most of the chemical reactions that involve halogens are oxidation-reduction reactions in aqueous solution. The halogens often form single bonds, when in the -1 oxidation state, with carbon or nitrogen in organic compounds. When a halogen atom is substituted for a covalently-bonded hydrogen atom in an organic compound, the prefix halo- can be used in a general sense, or the prefixes fluoro-, chloro-, bromo-, or iodo- can be used for specific halogen substitutions. Halogen elements can cross-link to form diatomic molecules with polar covalent single bonds.
Chlorine (Cl2) was the first halogen to be discovered in 1774, followed by iodine (I2), bromine (Br2), fluorine (F2), and astatine (At, discovered last in 1940). The name 'halogen' is derived from the Greek roots hal- ('salt') and -gen ('to form'). Together these words combine to mean 'salt former', referencing the fact that halogens form salts when they react with metals. Halite is the mineral name for rock salt, a natural mineral consisting essentially of sodium chloride (NaCl). Lastly, the halogens are also relevant in daily life, whether it be the fluoride that goes in toothpaste, the chlorine that disinfects drinking water, or the iodine that facilitates the production of thyroid hormones in one's body.
Elements
Fluorine - Fluorine has an atomic number of 9 and is denoted by the symbol F. Elemental fluorine was first discovered in 1886 by isolating it from hydrofluoric acid. Fluorine exists as a diatomic molecule in its free state (F2) and is the most abundant halogen found in the Earth's crust. Fluorine is the most electronegative element in the periodic table. It appears as a pale yellow gas at room temperature. Fluorine also has a relatively small atomic radius. Its oxidation state is always -1 except in its elemental, diatomic state (in which its oxidation state is zero). Fluorine is extremely reactive and reacts directly with all elements except helium (He), neon (Ne) and argon (Ar). In H2O solution, hydrofluoric acid (HF) is a weak acid. Although fluorine is highly electronegative, its electronegativity does not determine its acidity; HF is a weak acid due to the fact that the fluoride ion is basic (pH>7). In addition, fluorine produces very powerful oxidants. For example, fluorine can react with the noble gas xenon and form the strong oxidizing agent Xenon Difluoride (XeF2). There are many uses for fluorine, which will be discussed in Part VI of this article.
Chlorine - Chlorine has the atomic number 17 and the chemical symbol Cl. Chlorine was discovered in 1774 by extracting it from hydrochloric acid. In its elemental state, it forms the diatomic molecule Cl2. Chlorine exhibits multiple oxidation states, such as -1, +1, 3, 5, and 7. At room temperature it appears as a light green gas. Since the bond that forms between the two chlorine atoms is weak, the Cl2 molecule is very reactive. Chlorine reacts with metals to produce salts called chlorides. Chloride ions are the most abundant ions that dissolve in the ocean. Chlorine also has two isotopes: 35Cl and 37Cl. Sodium chloride is the most prevalent compound of the chlorides.
Bromine - Bromine has an atomic number of 35 with a symbol of Br. It was first discovered in 1826. In its elemental form, it is the diatomic molecule Br2. At room temperature, bromine is a reddish- brown liquid. Its oxidation states vary from -1, +1, 3, 4 and 5. Bromine is more reactive than iodine, but not as reactive as chlorine. Also, bromine has two isotopes: 79Br and 81Br. Bromine consists of bromide salts, which have been found in the sea. The world production of bromide has increased significantly over the years, due to its access and longer existence. Like all of the other halogens, bromine is an oxidizing agent, and is very toxic.
Iodine - Iodine has the atomic number 53 and symbol I. Iodine has oxidation states -1, +1, 5 and 7. Iodine exists as a diatomic molecule, I2, in its elemental state. At room temperature, it appears as a violet solid. Iodine has one stable isotope: 127I. It was first discovered in 1811 through the use of seaweed and sulfuric acid. Currently, iodide ions can be isolated in seawater. Although iodine is not very soluble in water, the solubility may increase if particular iodides are mixed in the solution. Iodine has many important roles in life, including thyroid hormone production. This will be discussed in Part VI of the text.
Astatine - Astatine is a radioactive element with an atomic number of 85 and symbol At. Its possible oxidation states include: -1, +1, 3, 5 and 7. It is the only halogen that is not a diatomic molecule and it appears as a black, metallic solid at room temperature. Astatine is a very rare element, so there is not that much known about this element. In addition, astatine has a very short radioactive half-life, no longer than a couple of hours. It was discovered in 1940 by synthesis. Also, it is thought that astatine is similar to iodine. However, these two elements are assumed to differ by their metallic character.
| Halogen | Electronic Configuration |
|---|---|
| Fluorine | 1s2 2s2 2p5 |
| Chlorine | [Ne]3s2 3p5 |
| Bromine | [Ar]3d104s24p5 |
| Iodine | [Kr]4d10 5s2 5p5 |
| Astatine | [Xe]4f14 5d10 6s2 6p5 |
Periodic Trends
The periodic trends observed in the halogen group:
Melting and Boiling Points (increases down the group)
The melting and boiling points increase down the group because of the van der Waals forces. The size of the molecules increases down the group. This increase in size means an increase in the strength of the van der Waals forces.
[F < Cl < Br < I < At]
| Halogen | Melting Point (˚C) | Boiling Point (˚C) |
|---|---|---|
| Fluorine | -220 | -188 |
| Chlorine | -101 | -35 |
| Bromine | -7.2 | 58.8 |
| Iodine | 114 | 184 |
| Astatine | 302 | 337 |
Atomic Radius (increases down the group)
The size of the nucleus increases down a group (F < Cl < Br < I < At) because the numbers of protons and neutrons increase. In addition, more energy levels are added with each period. This results in a larger orbital, and therefore a longer atomic radius.
| Halogen | Covalent Radius (pm) | Ionic (X-) radius (pm) |
|---|---|---|
| Fluorine | 71 | 133 |
| Chlorine | 99 | 181 |
| Bromine | 114 | 196 |
| Iodine | 133 | 220 |
| Astatine | 150 |
Ionization Energy (decreases down the group)
If the outer valence electrons are not near the nucleus, it does not take as much energy to remove them. Therefore, the energy required to pull off the outermost electron is not as high for the elements at the bottom of the group since there are more energy levels. Also, the high ionization energy makes the element appear non-metallic. Iodine and astatine display metallic properties, so ionization energy decreases down the group (At < I < Br < Cl < F).
| Halogen | First Ionization Energy (kJ/mol) |
|---|---|
| Fluorine | 1681 |
| Chlorine | 1251 |
| Bromine | 1140 |
| Iodine | 1008 |
| Astatine | 890±40 |
Electronegativity (decreases down the group)
The number of valence electrons in an atom increases down the group due to the increase in energy levels at progressively lower levels. The electrons are progressively further from the nucleus; therefore, the nucleus and the electrons are not as attracted to each other. An increase in shielding is observed. Electronegativity therefore decreases down the group (At < I < Br < Cl < F).
| Halogen | Electronegativity |
|---|---|
| Fluorine | 4.0 |
| Chlorine | 3.0 |
| Bromine | 2.8 |
| Iodine | 2.5 |
| Astatine | 2.2 |
Electron Affinity (decreases down the group)
Since the atomic size increases down the group, electron affinity generally decreases (At < I < Br < F < Cl). An electron will not be as attracted to the nucleus, resulting in a low electron affinity. However, fluorine has a lower electron affinity than chlorine. This can be explained by the small size of fluorine, compared to chlorine.
| Halogen | Electron Affinity (kJ/mol) |
|---|---|
| Fluorine | -328.0 |
| Chlorine | -349.0 |
| Bromine | -324.6 |
| Iodine | -295.2 |
| Astatine | -270.1 |
Reactivity of Elements (decreases down the group)
The reactivities of the halogens decrease down the group ( At < I < Br < Cl < F). This is due to the fact that atomic radius increases in size with an increase of electronic energy levels. This lessens the attraction for valence electrons of other atoms, decreasing reactivity. This decrease also occurs because electronegativity decreases down a group; therefore, there is less electron 'pulling.' In addition, there is a decrease in oxidizing ability down the group.
Hydrogen Halides and Halogen Oxoacids
Hydrogen Halides
A halide is formed when a halogen reacts with another, less electronegative element to form a binary compound. Hydrogen, for example, reacts with halogens to form halides of the form HX:
- Hydrogen Fluoride: HF
- Hydrogen Chloride: HCl
- Hydrogen Bromide: HBr
- Hydrogen Iodide: HI
Hydrogen halides readily dissolve in water to form hydrohalic (hydrofluoric, hydrochloric, hydrobromic, hydroiodic) acids. The properties of these acids are given below:
- The acids are formed by the following reaction: HX (aq) + H2O (l) → X- (aq) + H3O+ (aq)
- All hydrogen halides form strong acids, except HF
- The acidity of the hydrohalic acids increases as follows: HF < HCl < HBr < HI
Hydrofluoric acid can etch glass and certain inorganic fluorides over a long period of time.
It may seem counterintuitive to say that HF is the weakest hydrohalic acid because fluorine has the highest electronegativity. However, the H-F bond is very strong; if the H-X bond is strong, the resulting acid is weak. A strong bond is determined by a short bond length and a large bond dissociation energy. Of all the hydrogen halides, HF has the shortest bond length and largest bond dissociation energy.
Halogen Oxoacids
A halogen oxoacid is an acid with hydrogen, oxygen, and halogen atoms. The acidity of an oxoacid can be determined through analysis of the compound's structure. The halogen oxoacids are given below:
- Hypochlorous Acid: HOCl
- Chlorous Acid: HClO2
- Chloric Acid: HClO3
- Perchloric Acid: HClO4
- Hypobromous Acid: HOBr
- Bromic Acid: HBrO3
- Perbromic Acid: HBrO4
- Hypoiodous Acid: HOI
- Iodic Acid: HIO3
- Metaperiodic Acid: HIO4; H5IO6
In each of these acids, the proton is bonded to an oxygen atom; therefore, comparing proton bond lengths is not useful in this case. Instead, electronegativity is the dominant factor in the oxoacid's acidity. Acidic strength increases with more oxygen atoms bound to the central atom.
States of Matter at Room Temperature
| States of Matter (at Room Temperature) | Halogen | Appearance |
|---|---|---|
| Solid | Iodine | Violet |
| Astatine | Black/Metallic [Assumed] | |
| Liquid | Bromine | Reddish-Brown |
| Gas | Fluorine | Pale Yellow-Brown |
| Chlorine | Pale Green |
Explanation for Appearance
The halogens' colors are results of the absorption of visible light by the molecules, which causes electronic excitation. Fluorine absorbs violet light, and therefore appears light yellow. Iodine, on the other hand, absorbs yellow light and appears violet (yellow and violet are complementary colors, which can be determined using a color wheel). The colors of the halogens grow darker down the group:
- Fluorine → pale yellow/brown
- Chlorine → pale green
- Bromine → red-brown
- www.crscientific.com/brominecell4.jpg
- Iodine → violet
- genchem.chem.wisc.edu/lab/PTL...ments/I/I.jpeg
- Astatine* → black/metallic
- www4.msu.ac.th/satit/studentP...t/astatine.jpg
In closed containers, liquid bromine and solid iodine are in equilibrium with their vapors, which can often be seen as colored gases. Although the color for astatine is unknown, it is assumed that astatine must be darker than iodine's violet (i.e. black) based on the preceding trend.
Oxidation States of Halogens in Compounds
As a general rule, halogens usually have an oxidation state of -1. However, if the halogen is bonded to oxygen or to another halogen, it can adopt different states: the -2 rule for oxygen takes precedence over this rule; in the case of two different halogens bonded together, the more electronegative atom takes precedence and adopts the -1 oxidation state.
Example 1.1: Iodine Chloride (ICl)
Chlorine has an oxidation state of -1, and iodine will have an oxidation of +1. Chlorine is more electronegative than iodine, therefore giving it the -1 oxidation state.
)
Oxygen has a total oxidation state of -8 (-2 charge x 4 atoms= -8 total charge). Hydrogen has a total oxidation state of +1. Adding both of these values together, the total oxidation state of the compound so far is -7. Since the final oxidation state of the compound must be 0, bromine's oxidation state is +7.
One third exception to the rule is this: if a halogen exists in its elemental form (X2), its oxidation state is zero.
| Halogen | Oxidation States in Compounds |
|---|---|
| Fluorine | (always) -1* |
| Chlorine | -1, +1, +3, +5, +7 |
| Bromine | -1, +1, +3, +4, +5 |
| Iodine | -1, +1,+5, +7 |
| Astatine | -1, +1, +3, +5, +7 |
Example 1.3: Fluorine
Why does fluorine always have an oxidation state of-1 in its compounds?
Solution
Electronegativity increases across a period, and decreases down a group. Therefore, fluorine has the highest electronegativity of all of the elements, indicated by its position on the periodic table. Its electron configuration is 1s2 2s2 2p5. If fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). Because fluorine has a high electronegativity, it can easily remove the desired electron from a nearby atom. Fluorine is then isoelectronic with a noble gas (with eight valence electrons); all its outermost orbitals are filled. Fluorine is much more stable in this state.
Applications of Halogens
Fluorine: Although fluorine is very reactive, it serves many industrial purposes. For example, it is a key component of the plastic polytetrafluoroethylene (called Teflon-TFE by the DuPont company) and certain other polymers, often referred to as fluoropolymers. Chlorofluorocarbons (CFCs) are organic chemicals that were used as refrigerants and propellants in aerosols before growing concerns about their possible environmental impact led to their discontinued use. Hydrochlorofluorocarbons (HFCs) are now used instead. Fluoride is also added to toothpaste and drinking water to help reduce tooth decay. Fluorine also exists in the clay used in some ceramics. Fluorine is associated with generating nuclear power as well. In addition, it is used to produce fluoroquinolones, which are antibiotics. Below is a list of some of fluorine's important inorganic compounds.
| Compound | Uses |
|---|---|
| Na3AlF6 | Manufacture of aluminum |
| BF3 | Catalyst |
| CaF2 | Optical components, manufacture of HF, metallurgical flux |
| ClF3 | Fluorinating agent, reprocessing nuclear fuels |
| HF | Manufacture of F2, AlF3, Na3AlF6, and fluorocarbons |
| LiF | Ceramics manufacture, welding, and soldering |
| NaF | Fluoridating water, dental prophylaxis, insecticide |
| SF6 | Insulating gas for high-voltage electrical equipment |
| SnF2 | Manufacture of toothpaste |
| UF6 | Manufacture of uranium fuel for nuclear reactors |
Chlorine: Chlorine has many industrial uses. It is used to disinfect drinking water and swimming pools. Sodium hypochlorite (NaClO) is the main component of bleach. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. Chlorine is also present in polyvinyl chloride (PVC), and several other polymers. PVC is used in wire insulation, pipes, and electronics. In addition, chlorine is very useful in the pharmaceutical industry. Medicinal products containing chlorine are used to treat infections, allergies, and diabetes. The neutralized form of hydrochloride is a component of many medications. Chlorine is also used to sterilize hospital machinery and limit infection growth. In agriculture, chlorine is a component of many commercial pesticides: DDT (dichlorodiphenyltrichloroethane) was used as an agricultural insecticide, but its use was discontinued.
Bromine: Bromine is used in flame retardants because of its fire-resistant properties. It also found in the pesticide methyl bromide, which facilitates the storage of crops and eliminates the spread of bacteria. However, the excessive use of methyl bromide has been discontinued due to its impact on the ozone layer. Bromine is involved in gasoline production as well. Other uses of bromine include the production of photography film, the content in fire extinguishers, and drugs treating pneumonia and Alzheimer's disease.
Iodine: Iodine is important in the proper functioning of the thyroid gland of the body. If the body does not receive adequate iodine, a goiter (enlarged thyroid gland) will form. Table salt now contains iodine to help promote proper functioning of the thyroid hormones. Iodine is also used as an antiseptic. Solutions used to clean open wounds likely contain iodine, and it is commonly found in disinfectant sprays. In addition, silver iodide is important for photography development.
Astatine: Because astatine is radioactive and rare, there are no proven uses for this halogen element. However, there is speculation that this element could aid iodine in regulating the thyroid hormones. Also, 211At has been used in mice to aid the study of cancer.
VII. Outside Links
- Grube, Karl; Leffler, Amos J. 'Synthesis of metal halides (ML).' J. Chem. Educ.1993, 70, A204.
- This video provides information about some of the physical properties of chlorine, bromine, and iodine:http://www.youtube.com/watch?v=yP0U5rGWqdg
- The following video compares four halogens: fluorine, chlorine, bromine and iodine in terms of chemical reactions and physical properties. http://www.youtube.com/watch?v=u2ogMUDBaf4
- Color wheel referenced to in the text: http://www.wou.edu/las/physci/ch462/c-wheel.gif
- Elson, Jesse. 'A bonding parameter. III, Water solubilities and melting points of the alkali halogens.' J. Chem. Educ.1969, 46, 86.
- Fessenden, Elizabeth. 'Structural chemistry of the interhalogen compounds.' J. Chem. Educ. 1951, 28, 619.
- Holbrook, Jack B.; Sabry-Grant, Ralph; Smith, Barry C.; Tandel, Thakor V. 'Lattice enthalpies of ionic halides, hydrides, oxides, and sulfides: Second-electron affinities of atomic oxygen and sulfur.' J. Chem. Educ. 1990, 67, 304.
- Kildahl, Nicholas K. 'A procedure for determining formulas for the simple p-block oxoacids.' J. Chem. Educ. 1991, 68, 1001.
- Liprandi, Domingo A.; Reinheimer, Orlando R.; Paredes, José F.; L'Argentière, Pablo C. 'A Simple, Safe Way To Prepare Halogens and Study Their Visual Properties at a Technical Secondary School.' J. Chem. Educ. 1999 76.
- Meek, Terry L. 'Acidities of oxoacids: Correlation with charge distribution.'J. Chem. Educ. 1992, 69, 270.
Practice Problems
- Why does fluorine always have an oxidation state of -1 in its compounds?
- Find the oxidation state of the halogen in each problem:
- HOCl
- KIO3
- F2
- What are three uses of chlorine?
- Which element(s) exist(s) as a solid in room temperature?
- Do the following increase or decrease down the group of halogens?
- boiling point and melting point
- electronegativity
- ionization energy
Answers
- Electronegativity increases across a period, and decreases down a group. Therefore, fluorine has the highest electronegativity out of all of the elements. Because fluorine has seven valence electrons, it only needs one more electron to acheive a noble gas configuration (eight valence electrons). Therefore, it will be more likely to pull off an electron from a nearby atom.
- disinfecting water, pesticides, and medicinal products
- +1 (Hydrogen has an oxidation state of +1, and oxygen has an oxidation state of -2. Therefore, chlorine must have an oxidation state of +1 so that the total charge can be zero)
- +5 (Potassium's oxidation state is +1. Oxygen has an oxidation state of -2, so for this compound it is -6 (-2 charge x 3 atoms= -6). Since the total oxidation state has to be zero, iodine's oxidation state must be +5).
- 0 (Elemental forms always have an oxidation state of 0.)
- iodine and astatine
- increases
- decreases
- decreases
References
- Hill, Graham, and John Holman. Chemistry in Context. 5th ed. United Kingdom: Nelson Thornes, 2000. 224-25.
- Petrucci, Ralph H. Genereal Chemistry: Principles and Modern Applications. 9th Ed. New Jersey: Pearson Education Inc, 2007. 920-928.
- Verma, N.K., B. Kapila, and S.K. Khanna. Comprehensive Chemistry XII. New Delhi: Laxmi Publications, 2007. 718-30.
